The National Agency for Food and Drug Administration and Control (NAFDAC) has alerted Nigerians, including healthcare providers, about a counterfeit cancer treatment drug, Phesgo 600mg/600mg/10ml, labelled with batch number C5290S20.
In a public alert (No. 051/2024) available on the agency’s website, NAFDAC reported that the Marketing Authorisation Holder (MAH), Roche, received a complaint from a doctor at the Lagos University Teaching Hospital (LUTH-NSIA) regarding the suspected counterfeit product, which had not been administered, but matched the characteristics of a previously reported counterfeit batch, C3809C51.
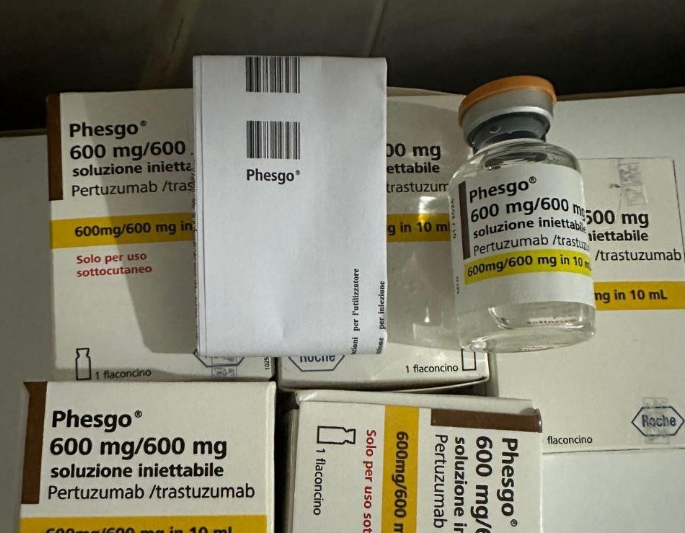
NAFDAC explained that though Phesgo 600mg/600mg Solution for Injection is used to treat breast cancer, as it works by killing cancer cells and preventing their further growth, the illegal marketing of counterfeit medicines was a serious health risk, as these products might not comply with regulatory standards and could undermine safety, quality, and effectiveness.
The agency therefore instructed all NAFDAC zonal directors and state coordinators to carry out surveillance and remove counterfeit products from their zones and states.
READ MORE STORIES https://classic97.net/onion-farmers-link-price-hike-and-scarcity-to-flood-climate-change/








